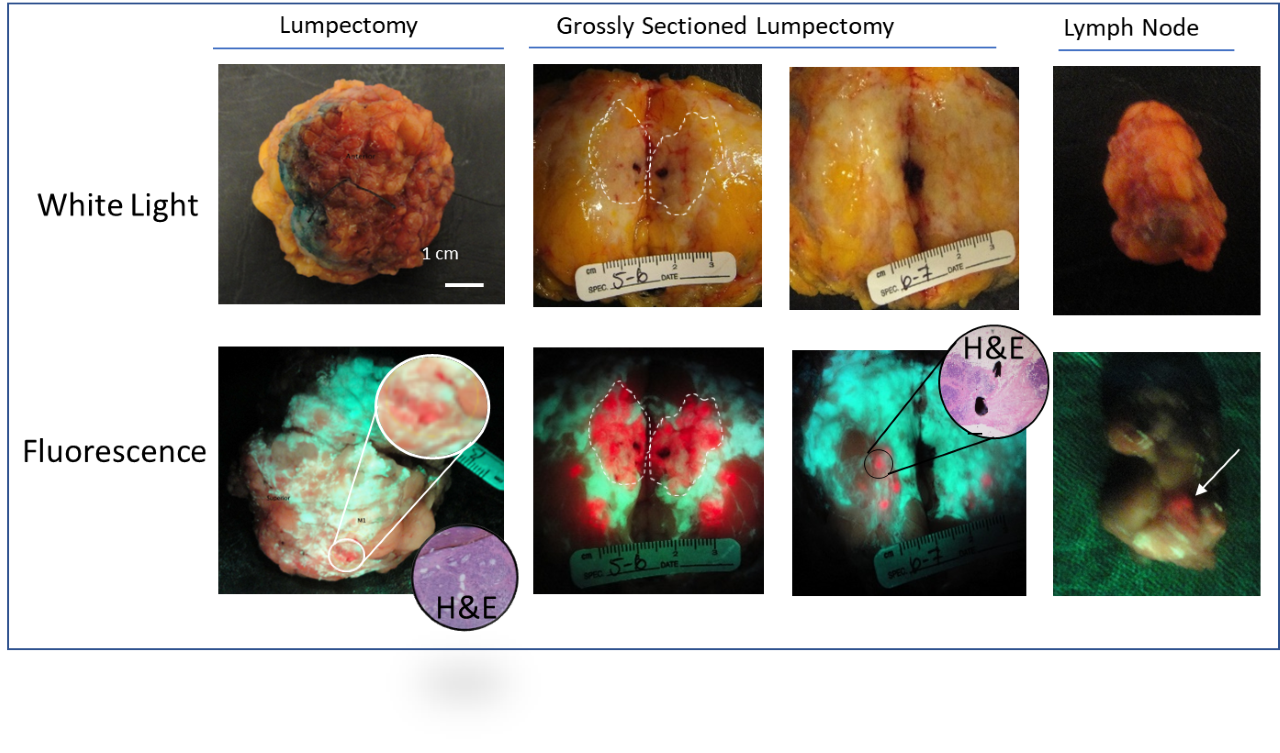
In 2009, the first patient was enrolled at Princess Margaret Cancer Center in this Phase II randomized controlled trial led by Dr. DaCosta in collaboration with surgeons Drs. Wey Leong and Alexandra Easson and pathologist Dr. Susan Done. The goal was to evaluate the clinical safety, feasibility and diagnostic performance of the PRODIGI handheld fluorescence imaging device combined with 5-ALA HCl for intraoperative visualization of invasive breast carcinomas. The Phase II trial was supported in large part by a $3M CIHR grant awarded to the DaCosta lab.
Today, over a decade later, the manuscript for this study was accepted for publication in Breast Cancer Research. This publication is the clinical report of visualization of 5-ALA HCl-induced fluorescence in breast carcinomas, including invasive lobular and ductal carcinoma in situ using the original DaCosta lab prototype handheld intraoperative fluorescence imaging device called “PRODIGI”. After many years, the PRODIGI device has evolved into a state-of-the-art fully handheld intraoperative fluorescence imaging technology called “Eagle”.
Currently, the collaboration with Drs. Leong, Easson, Done and others at the Princess Margaret Cancer Center has evolved into a new clinical trial called “RESTART” at Princess Margaret Cancer Center. This trial will test 5-ALA with a the Eagle imaging technology to study the biological mechanisms of 5-ALA-induced PpIX fluorescence specificity across various breast cancer subtypes in contrast with healthy tissues. (ClinicalTrials.gov Identifier: NCT01837225).
The DaCosta lab recognizes the incredible collaborative spirit of PMCC clinicians that helps to drive translation (cancer imaging) research forward to the international stage. Without the excitement and durable support of our clinician partners, we would not have been able to reach these important milestones as we try to overcome the challenges of cancer surgery for patients around the globe.
