Our clinical research program develops new imaging technologies and guides them from proof-of-concept towards first-in-human clinical feasibility studies.
The DaCosta Lab is focused on bringing innovative solutions to clinical problems with the goal of improving patient care and outcomes. We rely on our clinical partners to frame the problems patients and clinicians experience today and look to offer effective, easily implementable solutions. The DaCosta lab is focused on developing diagnostic, treatment monitoring and guidance imaging tools for use in clinics and operating rooms.

Clinical Research Projects
(clinicaltrials.gov ID NCT01837225)
Breast conserving surgery (BCS) is the most common primary treatment for patients with early invasive breast cancer. The goal of BCS is to resect and remove the tumor while conserving as much of the surrounding normal tissue as possible. Currently, there is no way for surgeons to determine the adequacy of surgical resection in real-time and the assessment of surgical margins requires histological examination which occurs days after the operation. In Canada, the average re-operation rate following BCS is 23%, with significant variability across provinces.
We are currently evaluating the PRODIGI™ device in an ongoing Phase II study involving 45 patients with primary invasive breast cancer undergoing lumpectomy or mastectomy as first line treatment. This fluorescence imaging device will be used in combination with the tumor localizing prodrug, 5-aminolevulinic acid (5-ALA). 5-ALA is a naturally occurring molecule in the heme biosynthesis pathway which, when exogenously administered, leads to preferential accumulation of the fluorescent heme precursor, protoporphyrin IX (PpIX), in tumor cells. When excited with 405 nm light, PpIX produces a red fluorescence emission (630 nm) which can be detected in real-time by the PRODIGI device. The primary objective of the trial is to clinically evaluate the combination of PRODIGI™ and pro-drug 5-ALA as a tumor contrast agent to improve the surgeons’ ability to differentiate breast tumor tissue from normal tissues during BSC.
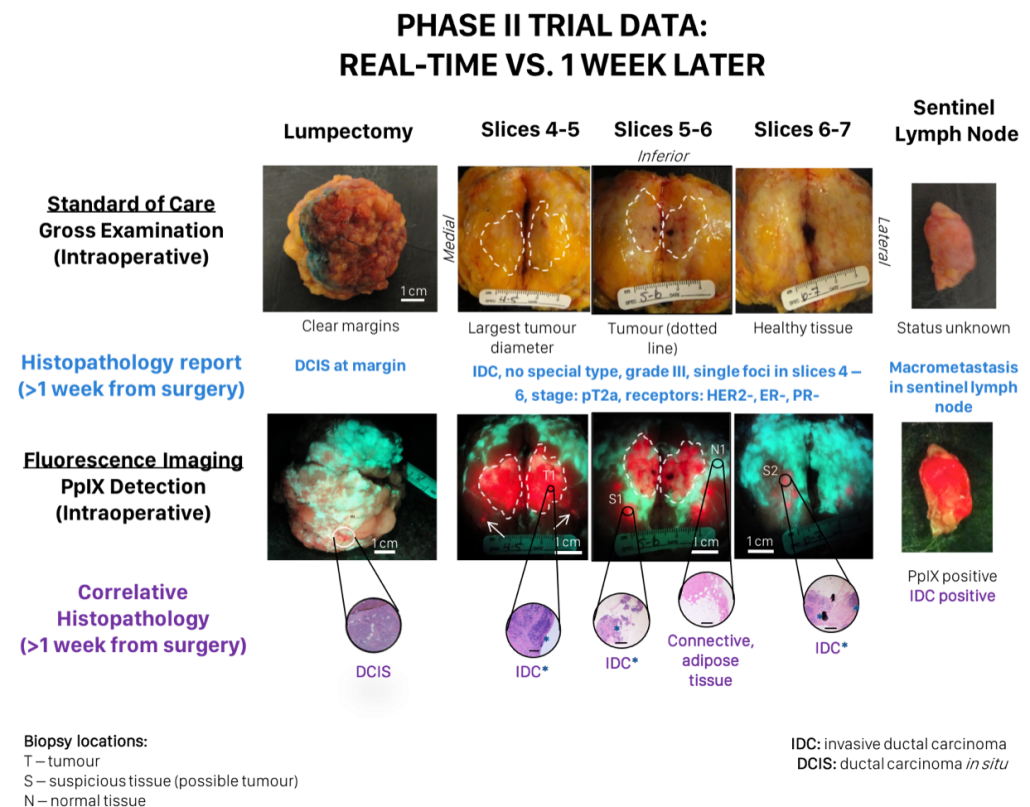
In our clinical trials we have demonstrated the proof-of-concept to image breast tumor margins intraoperatively based on 5-ALA-induced PpIX fluorescence in resected tissues (clinicaltrials.gov ID NCT01837225). Clinical user feedback from this ongoing trial has informed the development of an improved fluorescence imaging prototype device. Using this prototype, we are focused on defining specifications and device characteristics, including the required optical power output, illumination uniformity, and resolution. In parallel, we are also developing an optimized form factor into which our prototype can be miniaturized according the clinical feedback. These studies will be conducted in phantoms and on ex vivo lumpectomy samples from patients and will ultimately define the specifications required for a trial-ready device.
The new device will be validated and then tested in a pan-Canadian multicentre randomized clinical trial (“The Canadian FIGHT Breast Cancer Surgical Trial”; PI: R. DaCosta). Fourteen surgeons across Canada will test the device by examining tumor margins in 700+ patients. In their early stages, our prototypes provide a number of benefits over competing technologies. We anticipate the results of these studies will elucidate the clinical applicability of intraoperative fluorescence image guidance for BCS.
(clinicaltrials.gov ID NCT04163055)
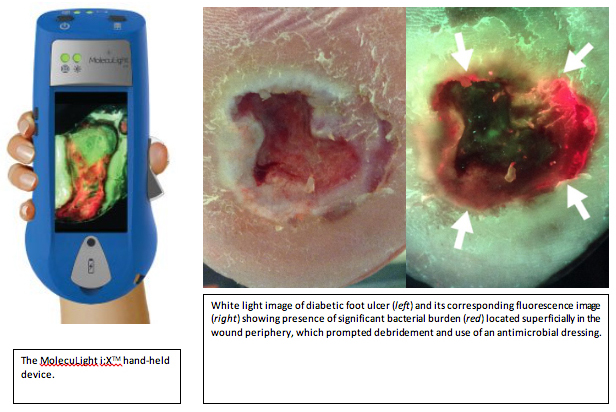
Standard-of-care (SoC) of diabetic foot ulcer infections involves visual inspection of the wound under white-light and identification of common signs of infection. However, SoC is limited by inconsistent guidelines, subjective assessment, and inaccurate debridement. To address these limitations, the DaCosta lab developed a handheld device (MolecuLight i:XTM) that can detect the presence of bacteria in wounds through autofluorescence in real-time, without making contact with the wound, and without the use of dyes or contrast agents. We will conduct a 3-year randomized control trial to test the therapeutic benefit of autofluorescence (AF) image guided intervention on diabetic foot ulcer management using MolecuLight i:XTM.
Our primary objective is to determine if AF-guided diagnosis and wound bed preparation improve wound healing in diabetic foot ulcers compared to SoC alone. Our secondary objective is to determine if AF-guided intervention decreases the bioburden in wounds by evaluating bacterial load and diversity. Our tertiary objective is to determine if AF-guided intervention is associated with improved quality of life and with reduced treatment costs. Outcome measures include frequency of wound healing, wound healing rates, measures of wound size by blinded research staff, bacteria load and species diversity analysis by blinded microbiologists, patient quality of life assessment and wound-related costs calculations.
(clinicaltrials.gov ID NCT04180748)
The microbiome can affect skin health from the gut-skin axis, from environmental exposure, and topical treatments. Decreasing biodiversity of skin microbiota has been linked to inflammatory conditions, allergies and skin health. This trial will be a cross sectional study to survey healthy volunteers and measure the density and diversity of skin flora of varying skin types. Our aim is to determine if there are associations between the diversity and/or density of normal bacterial flora and (1) the different skin types; (2) the different Fitzpatrick skin types; (3) the number of skin products used daily representing time spent on skin health. In addition, this study will evaluate the potential of an autofluorescence image-guided device (MolecuLight i:X™) to capture differences in healthy human skin flora through autofluorescence.
We will use the MolecuLight i:X™ to compare the fluorescent characteristics of normal skin flora against 16S RNA analysis of the skin microbiome and traditional microbiology techniques. In addition, we will use non-selective agars to grow bacteria according to the spatial topography of the skin, using a tape stripping method, with lightly adhesive 3M™Tegaderm wound dressings. This will serve as a “map” for fluorescent images by which to compare fluorescent features to bacterial species.

