Our preclinical research program focuses on identifying new avenues to improve conventional and emerging therapies, with a focus on cancer.
The DaCosta research team develops new optical imaging tools, in the fields of intravital microscopy and molecular probes, which are used in specialized animal models of human cancers. These innovative tools enable us to visualize and quantify biological processes and therapy-induced changes in the living tumor. We capture a tumor’s dynamic microenvironment in vivo, over time, at single cell level producing award winning images.
Preclinical Research Programs
Tumor hypoxia develops due to uncontrollable cell proliferation, altered metabolism, and abnormal tumor blood vessels. The resulting reduction in transport of oxygen and nutrients to the tumor and its microenvironment is one of the main features of solid tumors and has been shown to correlate with poor prognosis of cancer patients. Using optically-enabled intravital imaging techniques in transparent window chamber animal models, our Terry Fox-funded research aims to develop a better understanding of hypoxia in pancreatic tumors and methods of measuring hypoxia to help to predict patients’ outcome.
We are currently investigating the effects of radiation treatment on human pancreatic tumors, their vasculature and microenvironment to improve our understanding of how these different components are influenced by radiation. We have demonstrated that a high single dose of irradiation significantly reduces vascular function in a transient manner, leading to the development of tumor hypoxia. This effect may negatively impact tumor response to radiation, thus opening possibilities to target these specific changes in the tumors and improve the overall therapeutic outcomes. Specifically, we will investigate novel treatment strategies using hypoxia-targeted drugs (e.g. TH-302) to overcome challenges associated with the treatment options that are currently available and improve the overall therapeutic outcomes. This collaborative, translationally-driven project has the potential to inform the design of new clinical trials at the Princess Margaret Hospital.
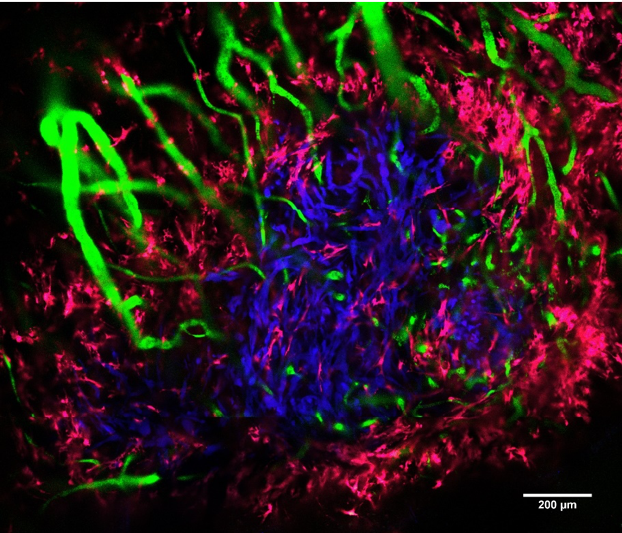
Pancreatic ductal adenocarcinoma (PDAC) has the lowest 5-year survival of any major cancer. Its highly aggressive and treatment-resistant phenotype often leads to early metastasis and poor outcomes. For patients with unresectable, locally advanced tumors (up to 50% of cases), local control remains a significant issue. Stereotactic body radiation therapy (SBRT) is an emerging treatment option for locally advanced PDAC. SBRT can provide significant advantages for patients’ quality of life over conventional chemoradiation, however, the rate of distant metastasis remains high.
Many studies show that the tumor microenvironment (i.e., stromal cells, blood vessels, and extracellular matrix) exerts a profound effect on the development of metastases and treatment resistance. In PDAC, this effect is especially true as it is characterized by a stroma-rich, hypoxic tumor microenvironment. The goal of this study is to investigate the effects of SBRT on the elements of the PDAC tumor microenvironment responsible for clinically relevant complications. We will use a novel mouse-imaging platform for longitudinal, intravital fluorescence microscopy to characterize the effect of SBRT on tumor vasculature and hypoxia, as well as pancreatic stellate cells and fibrillar collagen in the tumor. Observing how these stromal elements respond to SBRT will improve our understanding of how PDAC tumors develop treatment-resistance and subsequently invade the surrounding tissues.

This image was captured in a live mouse using a laser-scanning confocal microscope (LSM710, Zeiss). The pancreatic tumor (blue), blood vessels (green), and pancreatic stellate cells (pink) are visualized.
Nanosurfaces have improved clinical success rate of implants by increasing bone to implant contact. Currently there is lack of detailed understanding on the healing mechanism at the cellular level. Neovascularization is considered an essential prerequisite to osteogenesis, but no previous reports have examined the effect of implant surface topography on the spatiotemporal pattern of neovascularization during peri-implant healing.
We have developed a cranial window model to study peri-implant healing intravitally over clinically relevant time scales as a function of implant surface design. Quantitative intravital confocal imaging revealed that changing the surface topography of implants significantly affects peri-implant neovascularization. We are currently investigating the influence of vascular changes on other cascades involved in wound healing, including progenitor cell migration and immune response. Once we understand the healing mechanism under healthy conditions, we can improve the clinical efficacy of next-generation implants in cure-limiting diseased conditions.
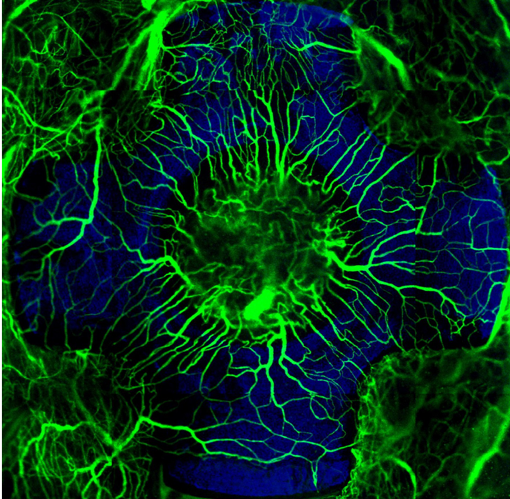
Blood vessels forming on a nano-surfaced implant. The radial arrangement of fluorescently labeled neovascularization (green), established during early endosseous healing associated with a nano-topographically complex cruciate titanium implant (blue), as visualized by intravital microscopy using a new cranial Implant window chamber model.
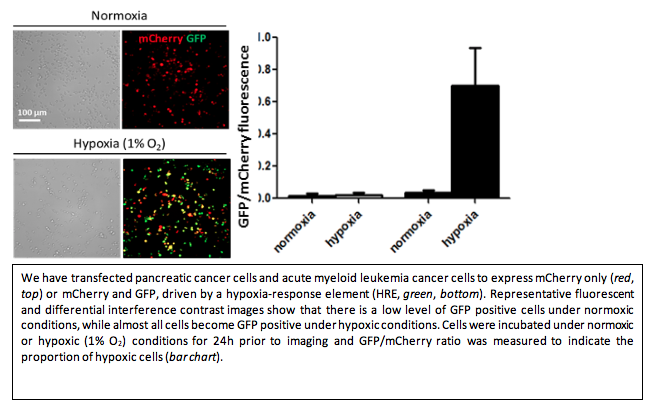
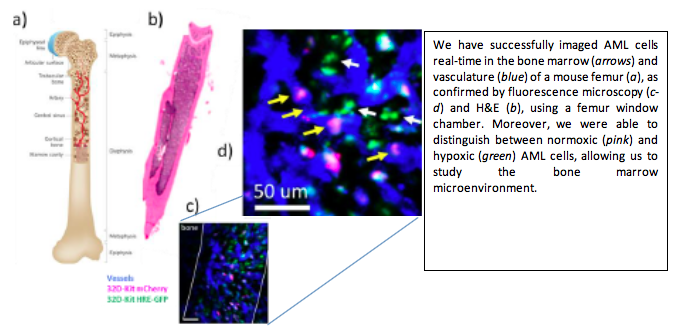
Acute myeloid leukemia (AML) is a disease of the bone marrow (BM) microenvironment, involving the growth of malignant cells close to other cells of the BM. Current animal studies focus on administering treatment and evaluating the effect at a later time, with minimal knowledge about what occurs between these two time points. Using two innovative and complementary experimental methods developed by Drs. DaCosta and Minden’s teams, our project is able to transform the way we investigate the development of AML in vivo in animal models of the disease.
The ability to observe in real-time the behavior of different cell types within the “living” BM can provide novel insights into the effect of the treatment and enable the development of new (immuno)therapies against this disease. Results obtained in this study have the potential to guide clinical trials at Princess Margaret involving the use of autologous IL-12 expressing AML cells and allogeneic double negative T cells (DNT) in patients at high risk of relapse. The experimental tools developed in this unique bench-to-bedside collaboration will also open up new avenues for basic research in leukemia, which could help overcome previous technological barriers in the field.
Biofilms are complex, organised communities of bacteria. Individual cells sense and communicate with other nearby cells to form a network in which they can share resources and defend from chemical and physical interference. Although some of these biofilms do exist symbiotically, the results of biofilm infection can cause devastating effects on the human body and subsequently on the health care industry. Clinical signs and symptoms are the primary tools to identify biofilms in wounds, however these tools are unspecific and require prolonged infection in patients.
We propose to use the fluorescence imaging device MolecuLight i:X, to monitor wound status, and investigate the differences in biofilm and infection autofluorescence in diabetic foot ulcers. This study will require the use of punch biopsies and histological staining to identify a biofilm based on phenotypic properties and to inform images of wounds containing suspected biofilm. Our future aim is to develop methods to locate biofilm and planktonic infection at the point of care using fluorescence imaging and digital solutions.
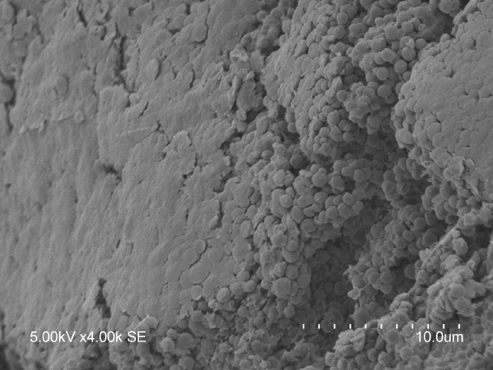
Image of suspected S. aureus biofilm grown on an ex vivo wound model and captured with a scanning electron microscope. The sticky extracellular matrix occludes the S. aureus cocci at the biofilm surface and can be seen between cells, like glue, holding them together.
Medical clinics rely on the interpretive results of microbiology laboratories in order to determine the diagnosis and appropriate treatment for patients with suspected infections. Antimicrobial resistant (AMR) bacterial species such as methicillin-resistant Staphylococcus aureus have increased in frequency due to misuse or over prescription of antibiotics, making the interpretive results of the microbiology team vital to determine the appropriate antimicrobial. However, laboratory testing requires 3-7 days before it can be reviewed by the physician. Thus, many patients, once prescribed an antibiotic at their primary visit to a doctor, may not receive their laboratory results until after finishing a course of broad-spectrum antibiotics.
Therefore, we propose using a Health Canada and FDA approved imaging device, MolecuLight i:X, at point of care to identify bacterial species and susceptibility to reduce the frequency of prescription of broad-spectrum antibiotics in chronic wound care. We propose a multi-step bacterial resistance and identification assay which can purify and identify key bacteria as well as susceptibility/resistance patterns using patient wound swabs.
