Niloufar Khosravi, a DaCosta Lab PhD student alumna, published in Biomaterials today:
“New insights into spatio-temporal dynamics of mesenchymal progenitor cell ingress during peri implant wound healing: Provided by intravital imaging”.
Abstract
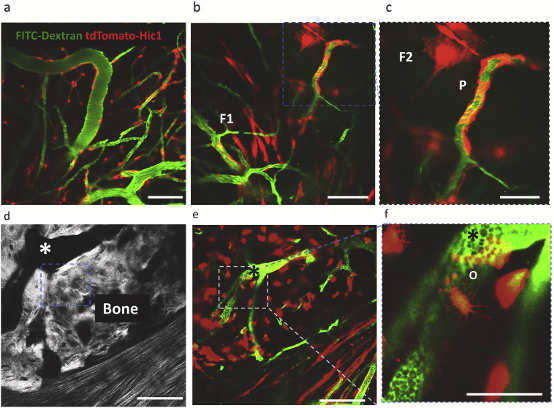
Surface topography drives the success of orthopedic and dental implants placed in bone, by directing the biology occurring at the tissue-implant interface. Over the last few decades, striking advancements have been made in the development of novel implant surfaces that enhance bone anchorage to their surfaces through contact osteogenesis: the combination of the two phenomena of recruitment and migration of mesenchymal progenitor cells to the implant surface, and their differentiation into bone-forming cells. While the latter is generally understood, the mechanisms and dynamics underlying the migration and recruitment of such progenitor cells into the wound site have garnered little attention. To address this deficit, we surgically inserted metallic implants with two different surface topographies into the skulls of mice, and then employed real-time spatiotemporal microscopic monitoring of the peri-implant tissue healing to track the ingress of cells. Our results show that nanotopographically complex, in comparison to relatively smooth, implant surfaces profoundly affect recruitment of both endothelial cells, which are essential for angiogenesis, and the mesenchymal progenitor cells that give rise to the reparative tissue stroma. The latter appear concomitantly in the wound site with endothelial cells, from the vascularized areas of the periosteum, and demonstrate a proliferative “bloom” that diminishes with time, although some of these cells differentiate into important stromal cells, pericytes and osteocytes, of the reparative wound. In separate experiments we show, using trajectory plots, that the directionality of migration for both endothelial and perivascular cells can be explained by implant surface dependent release of local cytokine gradients from platelets that would become activated on the implant surfaces during initial blood contact. These findings provide new biological insights into the earliest stages of wound healing, and have broad implications in the application of putative nano-topographically complex biomaterials in many tissue types.

