Ottolino-Perry K, Shahid A, DeLuca S, Son V, Liu Z, Rapic S, Thalanki Anantha N, Wang S, Chamma E, Blackmore K, Gibson C, Medeiros PJ, Majeed S, Chu A, Pizzolato A, Rosen CF, Lindvere-Teene L, Dunham D, Kulbatski I, Panzarella T, Done SJ, Easson AM, Leong WL, DaCosta RS; Proc. SPIE 11070, 17th International Photodynamic Association World Congress, 110701Y (14 August 2019)
doi.org/10.1117/12.2527216
Abstract
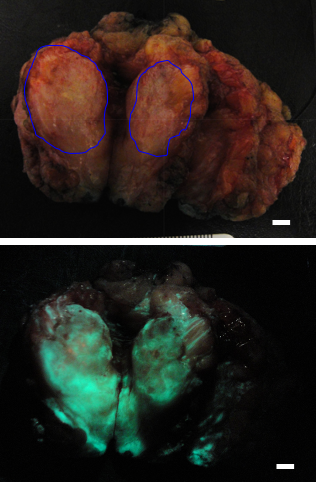
Re-excision due to inability to visualize positive margins following breast-conserving surgery is a significant clinical challenge. 5-aminolevulinic acid (5-ALA), a non-fluorescent prodrug, leads to intracellular accumulation of fluorescent porphyrins in tumor cells. This single-centre Phase II randomized controlled trial evaluated the clinical safety, feasibility and diagnostic performance of a new handheld fluorescence imaging device (PRODIGI) combined with 5-ALA hydrochloride (HCl) for intraoperative visualization of invasive breast carcinomas. Fifty-four patients were enrolled in the study and randomized to receive no 5-ALA HCl or orally administered 5-ALA HCl (15 mg/kg or 30 mg/kg BW). Forty-five patients (n = 15/group) were included in the analysis. Fluorescence imaging of the excised surgical specimen was performed and biopsies were collected from within and outside the clinically-demarcated tumor border for blinded histopathological analysis. In the absence of 5-ALA HCl, tissue autofluorescence imaging lacked tumor-specific fluorescent contrast. Administration of 5-ALA HCl resulted in tumors that fluoresced bright red with improved visualization of tumor contrasted against normal tissue autofluorescence. In the 15 mg/kg 5-ALA dose group the positive predictive value (PPV) for detecting tumor inside and outside the grossly demarcated tumor border was 100.0% and 55.6%, respectively and 100.0% and 50.0% respectively in the 30 mg/kg dose group. No drug or device-related adverse events were observed and technical feasibility and clinical integration of this intraoperative tumor visualization approach were confirmed. This is the first known clinical report of visualization of 5-ALA HCl-induced fluorescence in invasive lobular and ductal breast carcinoma using a real-time handheld intraoperative fluorescence imaging device.
