Stammes MA, Knol-Blankevoort VT, Cruz LJ, Feitsma HR, Mezzanotte L, Cordfunke RA, Sinisi R, Dubikovskaya EA, Maeda A, DaCosta RS, Bierau K, Chan A, Kaijzel EL, Snoeks TJ, van Beek ER, Löwik CW; Mol Imaging Biol. 2016 Dec;18(6):905-915
doi.org/10.1007/s11307-016-0972-7
Abstract
PURPOSE:
Recently we showed that a number of carboxylated near-infrared fluorescent (NIRF) cyanine dyes possess strong necrosis avid properties in vitro as well as in different mouse models of spontaneous and therapy-induced tumor necrosis, indicating their potential use for cancer diagnostic- and prognostic purposes. In the previous study, the detection of the cyanines was achieved by whole body optical imaging, a technique that, due to the limited penetration of near-infrared light, is not suitable for investigations deeper than 1 cm within the human body. Therefore, in order to facilitate clinical translation, the purpose of the present study was to generate a necrosis avid cyanine-based NIRF probe that could also be used for single photon emission computed tomography (SPECT). For this, the necrosis avid NIRF cyanine HQ4 was radiolabeled with 111indium, via the chelate diethylene triamine pentaacetic acid (DTPA).
PROCEDURES:
The necrosis avid properties of the radiotracer [111In]DTPA-HQ4 were examined in vitro and in vivo in different breast tumor models in mice using SPECT and optical imaging. Moreover, biodistribution studies were performed to examine the pharmacokinetics of the probe in vivo.
RESULTS:
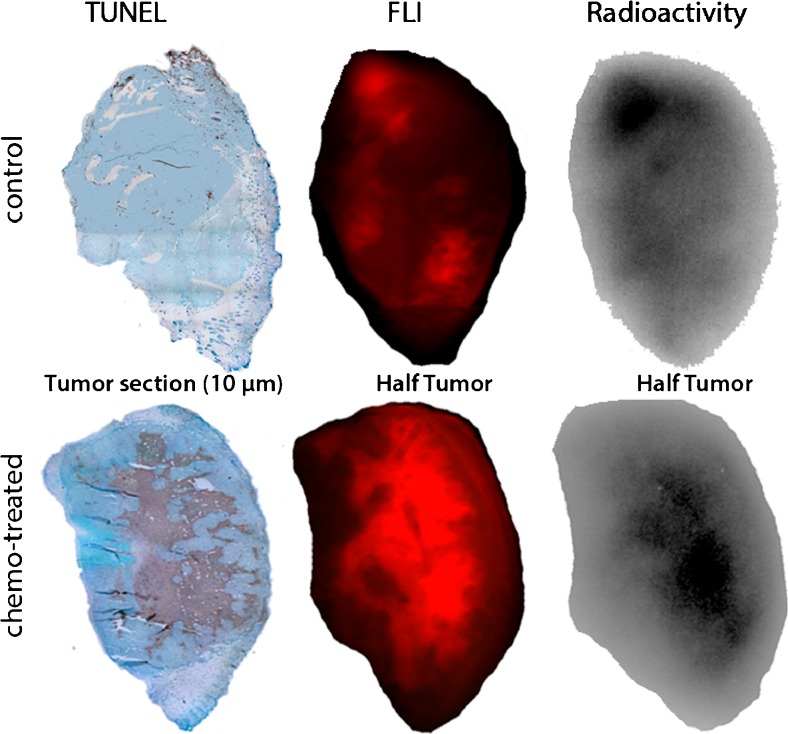
Using optical imaging and radioactivity measurements, in vitro, we showed selective accumulation of [111In]DTPA-HQ4 in dead cells. Using SPECT and in biodistribution studies, the necrosis avidity of the radiotracer was confirmed in a 4T1 mouse breast cancer model of spontaneous tumor necrosis and in a MCF-7 human breast cancer model of chemotherapy-induced tumor necrosis.
CONCLUSIONS:
The radiotracer [111In]DTPA-HQ4 possessed strong and selective necrosis avidity in vitro and in various mouse models of tumor necrosis in vivo, indicating its potential to be clinically applied for diagnostic purposes and to monitor anti-cancer treatment efficacy.