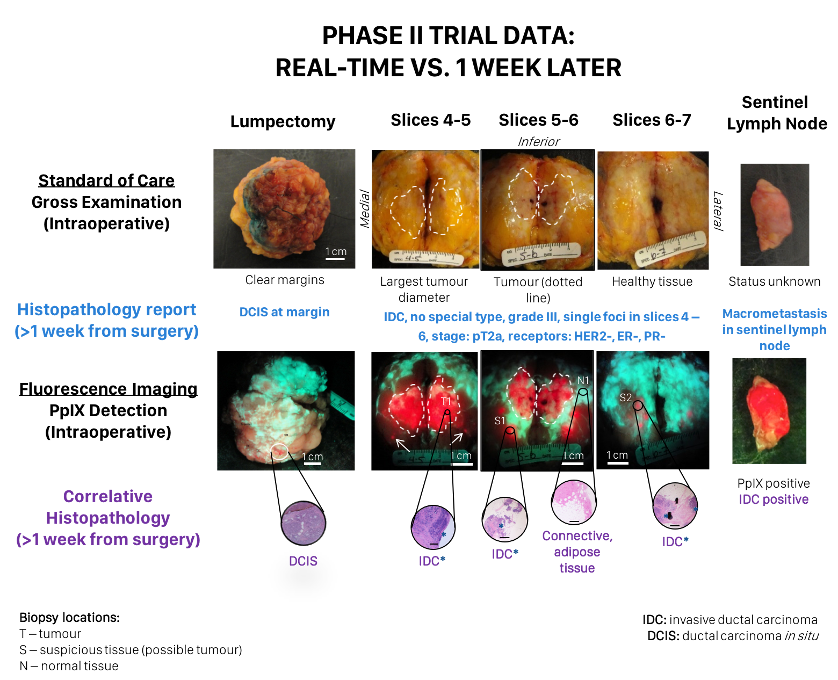
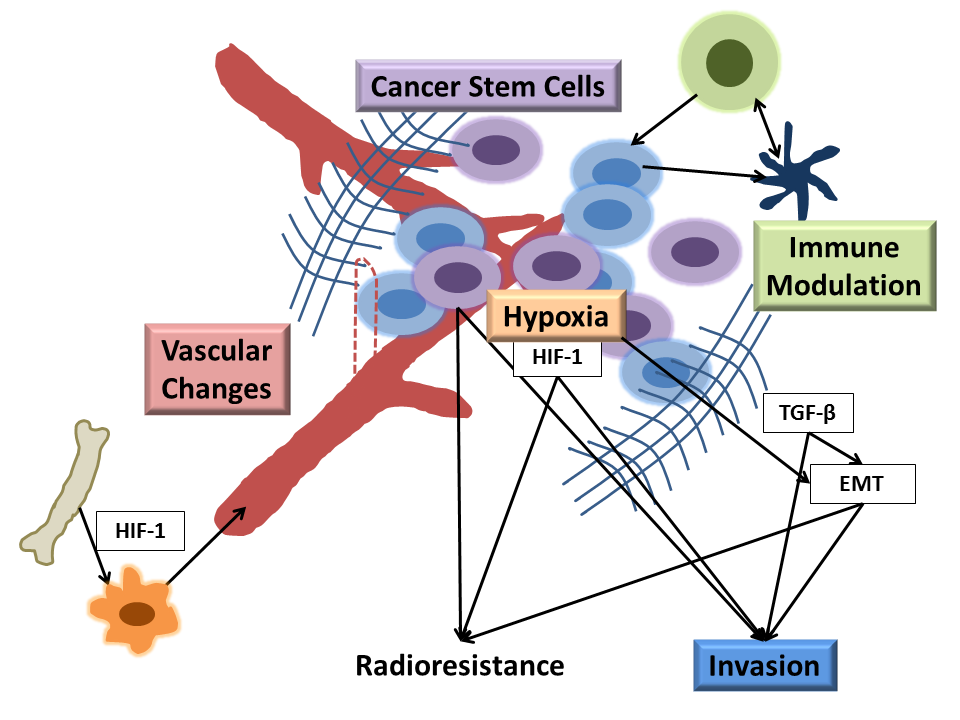
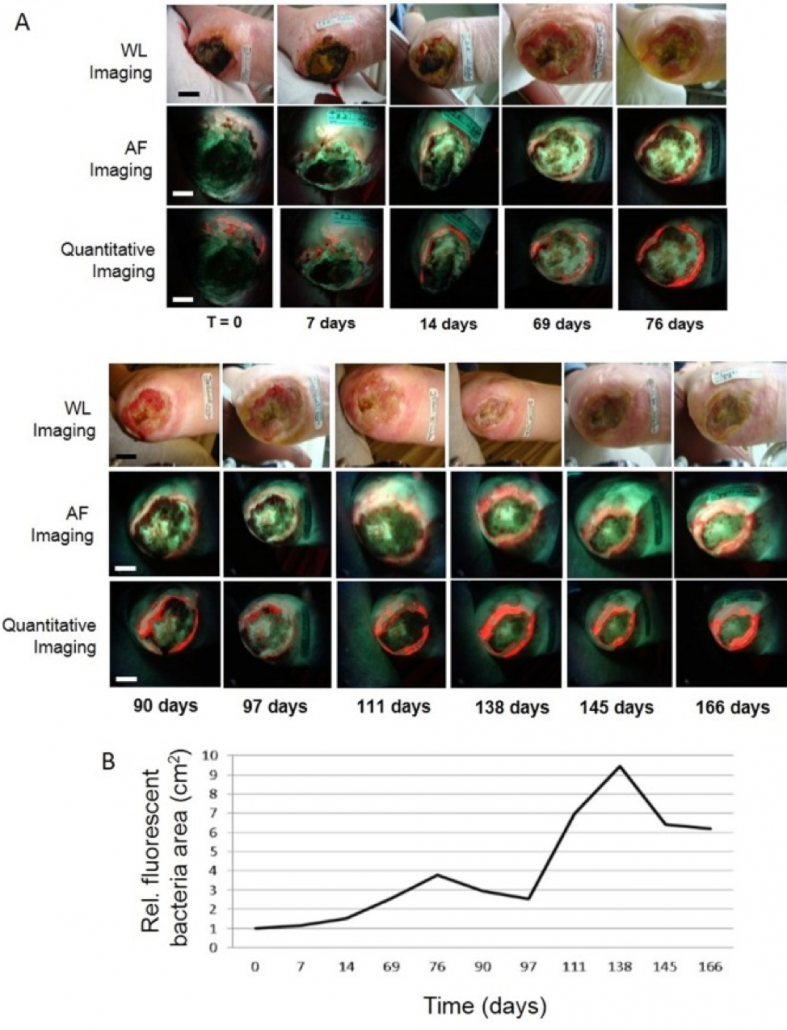
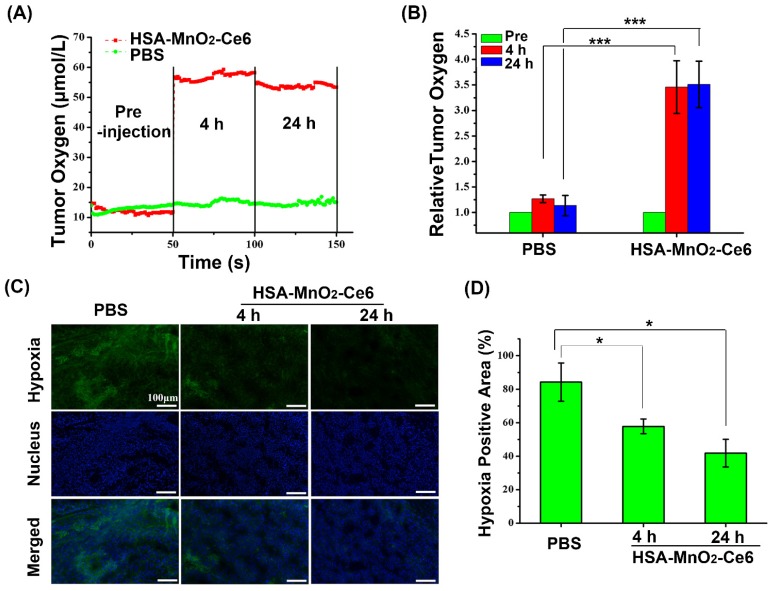
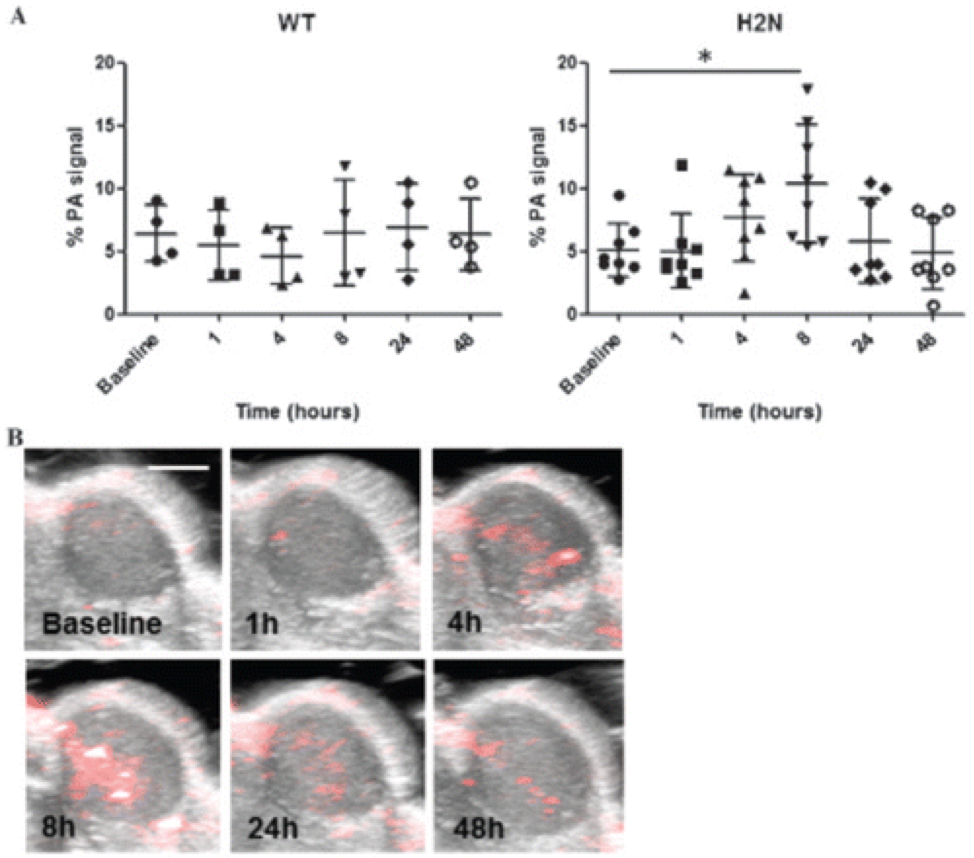
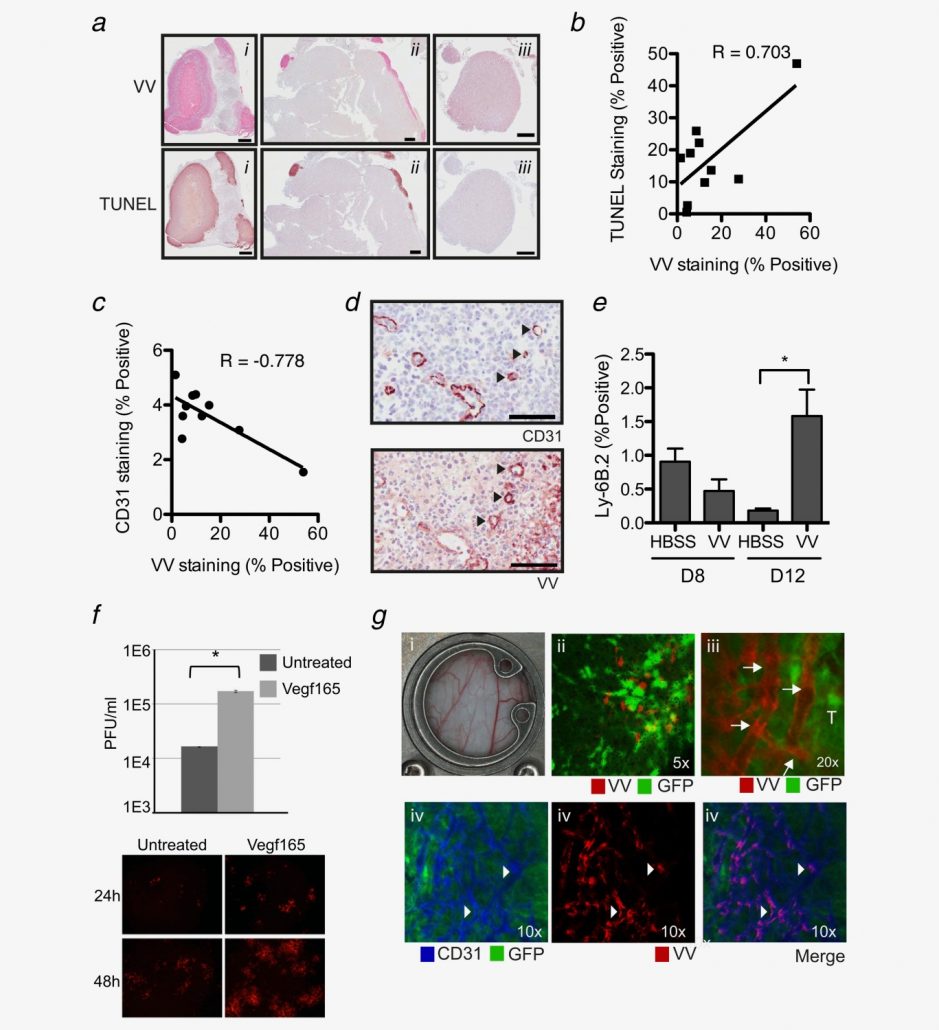
Assessing the Accuracy of Bioluminescence Image-Guided Stereotactic Body Radiation Therapy of Orthotopic Pancreatic Tumors Using a Small Animal Irradiator
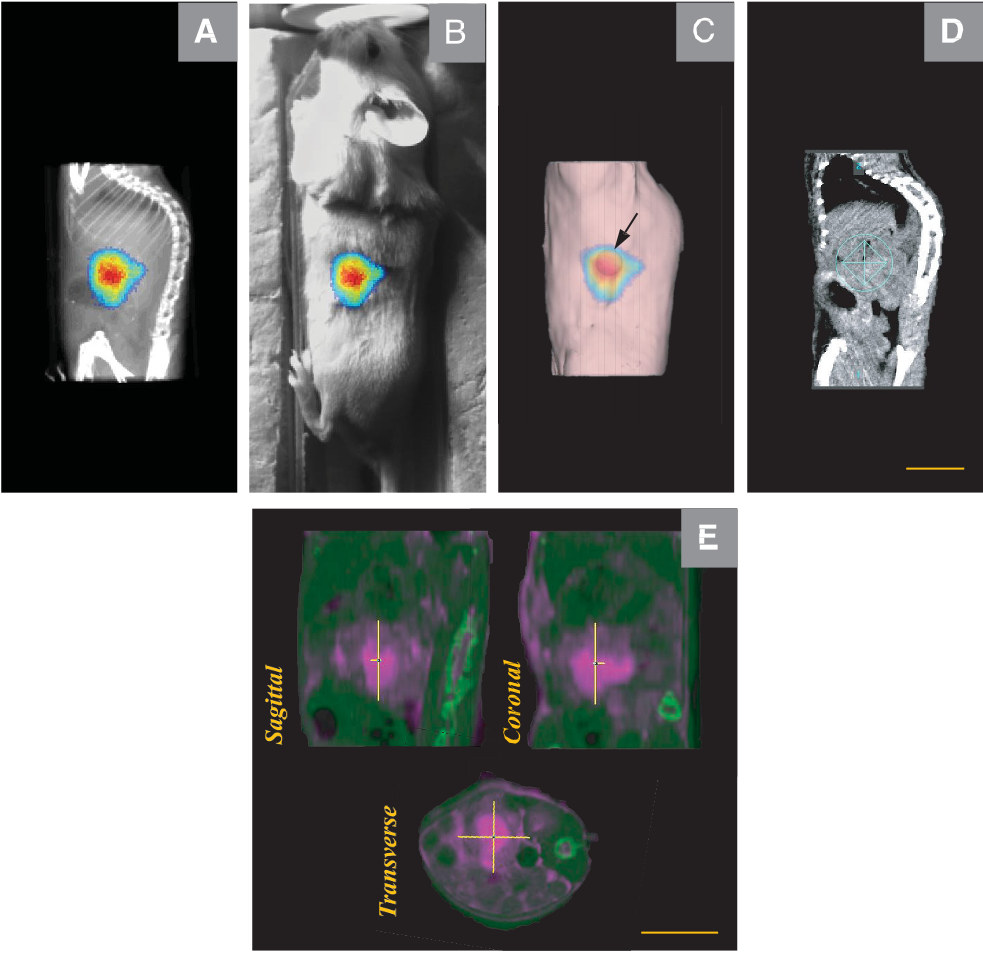
https://dacostalab.ca/wp-content/uploads/2022/04/RadiationResearch1.png 983 970 DaCosta Lab DaCosta Lab https://dacostalab.ca/wp-content/uploads/2022/04/RadiationResearch1.pngOur recent publication in Radiation Research, “Assessing the Accuracy of Bioluminescence Image-Guided Stereotactic Body Radiation Therapy of Orthotopic Pancreatic Tumors Using a Small Animal Irradiator” is a collaboration between the DaCosta Lab and Dr. Robert Weersink. In this preclincal study, Dr. Sara Rapic (former Post-doctoral fellow) and Timothy Samuel (Ph.D. candidate) demonstrated the improved accuracy of using 3-dimensional bioluminescence imaging (BLI) to deliver targeted high dose radiotherapy in orthotopic mouse models of pancreatic cancer. This BLI image-guided precision in the delivery of radiation therapy to pancreatic tumors in vivo ensures the precision of the treatment using a novel small animal irradiator. This methodology allows us to investigate the response of these tumors, their vasculature and microenvironment to radiation therapy tailored to simulate clinical radiotherapy regimes in patients. This technology is also helping to expand our understanding of tumor hypoxia in pancreatic cancer and how hypoxia can impact tumor response as well as modify the tumor microenvironment over time. Currently, this platform is being used to study the relationship between pancreatic tumor cell hypoxia and changes in tumor-associated stromal cells in vivo in the orthotopic setting by exploiting our intravital fluorescence imaging platform with and without radiation treatment. Representation of image collection workflow showing: CBCT (panel A), white light (panel B), and 3D surface mesh (panel C) images overlayed with the bioluminescence image of an anesthetized mouse previously injected with luciferin. The bioluminescence signal (red . High signal, blue . low signal) occurs from the pancreatic tumor, which cannot be seen in the CBCT image only. All images are co-registered automatically since the modules are integrated into the same gantry without the need to move the mouse. The optical point source (black arrow) is calculated from the bioluminescence signal and targeted (blue crosshairs) for radiation (panel D). MRI-guided targeting (panel E; yellow crosshairs) is done after co-registration of a sequentially acquired magnetic resonance (purple) and CBCT image (green). Although the same imaging bed is used, the mouse is allowed to wake up between the scans, requiring manual registration. Scale bars = 1 cm The X-RAD 225Cx’s capability to acquire bioluminescence images allows for functional analysis of tumor viability. Scaled bioluminescence images (normalized for exposure time) overlayed with their co-registered CBCT scan are shown for a representative animal in each group before treatment and when receiving their third fraction and last fraction (panel A). Panel B: Maximum photon counts after 5 fractions of SBRT (D5) for the control, BLI-guided, and MRI-guided SBRT groups. The optical signal was the lowest when BLI was used to guide the Xray dose to the tumor. When the X-ray dose was guided to the tumor in the magnetic resonance image co-registered with the CBCT scan, the optical signal was comparable to that of the untreated group (panel C) Tumor volume, as determined from the excised tumor after the last fraction using a digital caliper, did not differ between groups, but a slight reduction was observed in SBRT-treated groups, albeit not significantly.
read more